Maintaining Your Aquarium
|
Large volumes of water enable more stability in a tank by diluting effects from death or contamination. Any event that perturbs the system pushes an aquarium away from equilibrium; the more water that is contained in a tank, the easier such a systemic shock is to absorb, as the effects of that event are diluted. For example, the death of the only fish in a three U.S. gallon tank (11 L) causes dramatic changes in the system, while the death of that same fish in a 100 U.S. gallon (400 L) tank with many other fish in it represents only a minor change in the balance of the tank. For this reason, hobbyists often favor larger tanks, as they are more stable systems requiring less attention to the maintenance of equilibrium.
There are a variety of nutrient cycles that are important in the aquarium. Dissolved oxygen enters the system at the surface water-air interface or through the actions of an air pump. Carbon dioxide escapes the system into the air. The phosphate cycle is an important, although often overlooked, nutrient cycle. Sulfur, iron, and micronutrients also cycle through the system, entering as food and exiting as waste. Appropriate handling of the nitrogen cycle, along with supplying an adequately balanced food supply and considered biological loading, is enough to keep these other nutrient cycles in approximate equilibrium, but not forever.
The solute content of water is perhaps the most important aspect of water conditions, as total dissolved solids and other constituents can dramatically impact basic water chemistry, and therefore how organisms are able to interact with their environment. Salt content, or salinity, is the most basic classification of water conditions. An aquarium may have fresh water (salinity below 500 parts per million), simulating a lake or river environment; brackish water (a salt level of 500 to 30,000 PPM), simulating environments lying between fresh and salt, such as estuaries; and salt water or sea water (a salt level of 30,000 to 40,000 PPM), simulating an ocean or sea environment. Rarely, even higher salt concentrations are maintained in specialized tanks for raising brine organisms.
Saltwater is typically alkaline, while the pH (alkalinity or acidicity) of fresh water varies more. Hardness measures overall dissolved mineral content; hard or soft water may be preferred. Hard water is usually alkaline, while soft water is usually neutral to acidic. Dissolved organic content and dissolved gases content are also important factors.
Home aquarists typically use tap water supplied through their local water supply network to fill their tanks. Because of the concentration of chlorine used to disinfect drinking water supplies for human consumption, straight tap water cannot be used in the USA. In the past, it was possible to "condition" the water by simply letting the water stand for a day or two, which allows the chlorine time to dissipate. However, chloramine is now used more often as it is much stabler and will not leave the water as readily. Additives formulated to remove chlorine or chloramine are often all that is needed to make the water ready for aquarium use. Brackish or saltwater aquaria require the addition of a mixture of salts and other minerals, which are commercially available.
More sophisticated aquarists may make other modifications to their base water source to modify the water's alkalinity, hardness, or dissolved content of organics and gases, before adding it to their aquaria. This can be accomplished by additives, such as sodium bicarbonate, to raise pH. Some aquarists will filter or purify their water prior to adding it to their aquarium. There are two processes used: deionization or reverse osmosis. In contrast, public aquaria with large water needs often locate themselves near a natural water source (such as a river, lake, or ocean) in order to have access to water that does not require much further treatment.
The temperature of the water forms the basis of one of the two most basic aquarium classifications: tropical vs. cold water. Most fish and plant species tolerate only a limited range of water temperatures: Tropical or warm water aquaria, with an average temperature of about 25 °C (77 °F), are much more common. Cold water aquaria are those with temperatures below what would be considered tropical; some varieties of fish are better suited to this cooler environment. More important than the temperature range itself is the consistency in temperature; most organisms are not accustomed to sudden changes in temperatures, which could cause shock and lead to disease. Water temperature can be regulated with a combined thermostat and heater unit (or, more rarely, with a cooling unit).
Water movement can also be important in accurately simulating a natural ecosystem. Aquarists may prefer anything from still water up to swift simulated currents in an aquarium, depending on the conditions best suited for the aquarium's inhabitants. Water movement can be controlled through the use of aeration from air pumps, powerheads, and careful design of internal water flow (such as location of filtration system points of inflow and outflow).
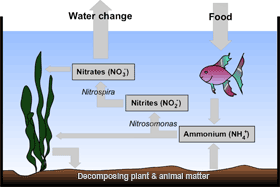
Of primary concern to the aquarist is management of the biological waste produced by an aquarium's inhabitants. Fish, invertebrates, fungi, and some bacteria excrete nitrogen waste in the form of ammonia (which will convert to ammonium, in acidic water) and must then pass through the nitrogen cycle. Ammonia is also produced through the decomposition of plant and animal matter, including fecal matter and other detritus. Nitrogen waste products become toxic to fish and other aquarium inhabitants at high concentrations.
The Process
A well-balanced tank contains organisms that are able to metabolize the waste products of other aquarium residents. The nitrogen waste produced in a tank is metabolized in aquaria by a type of bacteria known as nitrifiers (genus Nitrosomonas). Nitrifying bacteria capture ammonia from the water and metabolize it to produce nitrite. Nitrite is also highly toxic to fish in high concentrations. Another type of bacteria, genus Nitrospira, converts nitrite into nitrate, a less toxic substance to aquarium inhabitants. (Nitrobacter bacteria were previously believed to fill this role, and continue to be found in commercially available products sold as kits to "jump start" the nitrogen cycle in an aquarium. While biologically they could theoretically fill the same niche as Nitrospira, it has recently been found that Nitrobacter are not present in detectable levels in established aquaria, while Nitrospira are plentiful.) This process is known in the aquarium hobby as the nitrogen cycle.
In addition to bacteria, aquatic plants also eliminate nitrogen waste by metabolizing ammonia and nitrate. When plants metabolize nitrogen compounds, they remove nitrogen from the water by using it to build biomass that decays more slowly than ammonia-driven plankton already dissolved in the water.
Maintaining the nitrogen cycle
Although informally called the nitrogen cycle by hobbyists, it is in fact only a portion of a true cycle: nitrogen must be added to the system (usually through food provided to the tank inhabitants), and nitrates accumulate in the water at the end of the process, or become bound in the biomass of plants. This accumulation of nitrates in home aquaria requires the aquarium keeper to remove water that is high in nitrates, or remove plants which have grown from the nitrates.
Aquaria kept by hobbyists often do not have the populations of bacteria needed to detoxify nitrogen waste from tank inhabitants. This problem is most often addressed through two filtration solutions: Activated carbon filters absorb nitrogen compounds and other toxins from the water, while biological filters provide a medium designed for colonization by the desired nitrifying bacteria. Activated carbon and other substances, such as ammonia absorbing resines, will stop working when their pores get full, so these components have to be replaced with fresh stocks constantly.
New aquaria often have problems associated with the nitrogen cycle due to insufficient number of beneficial bacteria, known as "New Tank Syndrome". Therefore new tanks have to be matured before stocking them with fish. There are three basic approaches to this: the fishless cycle the silent cycle and slow growth.
No fish are kept in a tank undergoing a "fishless" cycle. Instead, small amounts of ammonia are added to the tank to feed the bacteria being cultured. During this process, ammonia, nitrite, and nitrate levels are tested to monitor progress. The "silent" cycle is basically nothing more than densely stocking the aquarium with fast-growing aquatic plants and relying on them to consume the nitrogen, allowing the necessary bacterial populations time to develop. According to anecdotal reports of aquarists specializing in planted tanks, the plants can consume nitrogenous waste so efficiently that the spikes in ammonia and nitrite levels normally seen in more traditional cycling methods are greatly reduced, if they are detectable at all. "Slow growth" entails slowly increasing the population of fish over a period of 6 to 8 weeks, giving bacteria colonies time to grow and stabilize with the increase in fish waste.
The largest bacterial populations are found in the filter; efficient filtration is vital. Sometimes, a vigorous cleaning of the filter is enough to seriously disturb the biological balance of an aquarium. Therefore, it is recommended to rinse mechanical filters in an outside bucket of aquarium water to dislodge organic materials that contribute to nitrate problems, while preserving bacteria populations. Another safe practice consists of cleaning only one half of the filter media every time the filter or filters are serviced.
Biological loading is a measure of the burden placed on the aquarium ecosystem by its living inhabitants. High biological loading in an aquarium represents a more complicated tank ecology, which in turn means that equilibrium is easier to perturb. In addition, there are several fundamental constraints on biological loading based on the size of an aquarium. The surface area of water exposed to air limits dissolved oxygen intake by the tank. The capacity of nitrifying bacteria is limited by the physical space they have available to colonize. Physically, only a limited size and number of plants and animals can be fit into an aquarium while still providing room for movement. Simply, all kinds of biology decay, and biological loading refers to that rate of decay in proportion to tank volume.
Calculating aquarium capacity
An aquarium can only support a certain number of fish. Limiting factors include the availability of oxygen in the water and the rate at which the filtration can process waste. Aquarists have developed rules of thumb to estimate the number of fish that can be kept in an aquarium; the examples below are for small freshwater fish, larger freshwater fishes and most marine fishes need much more generous allowances.
- 1 inch of fish length per gallon of water.
- 1 inch of fish length per 12 square inches of surface area.
Experienced aquarists warn against applying these rules too strictly because they do not consider other important issues such as growth rate, activity level, social behaviour, surface area of plant life, and so on. To some degree, establishing the maximum loading capacity of an aquarium depends upon slowly adding fish and monitoring water quality over time, essentially a trial and error approach.
Factors affecting capacity
Though many conventional methods of calculating the capacity of aquarium are based on volume and pure length of fish, there are other variables. One variable is differences between fish. Smaller fish consume more oxygen per gram of body weight than larger fish. Labyrinth fish, having the capability to breathe atmospheric oxygen, are noted for not needing as much surface area (however, some of these fish are territorial, and may not appreciate crowding). Barbs also require more surface area than tetras of comparable size.
Oxygen exchange at the surface is an important constraint, and thus the surface area of the aquarium. Some aquarists go so far as to say that a deeper aquarium with more volume holds no more fish than a shallower aquarium of the same surface area. The capacity can be improved by surface movement and water circulation such as through aeration, which not only improves oxygen exchange, but also the decomposition of waste materials.
The presence of waste materials presents itself as a variable as well. Decomposition in solution tends to consume oxygen. Oxygen dissolves less readily in warmer water; this is a double-edged sword as warmer temperatures make more active fish, which in turn consume even more oxygen. Stress due to temperature changes is especially obvious in coldwater aquaria where the temperature may swing from low temperatures to high temperatures on hotter days.
Many marine fish will eat Flake food, which you’ll be able to find at the fish store. Tetra is popular. You can also purchase frozen foods at the fish store to feed your fish. Remember, Tangs are herbivores, so they will not want meaty foods, but rather algae based foods. Frozen Spirulina Enriched Brine Shrimp, Frozen Mysis, Frozen Blood Worms, Frozen Plankton are all good choices, and you can rotate through these over the period of a week, mixing their diet nicely. These foods come in trays, frozen in cubes. One thawed cube is sufficient for a few fish, so avoid overfeeding. You should see all the food consumed in 5 minutes. If you see food after that, you’ve fed too much. Feeding once a day is enough, unless you have a Tang. Then feeding twice a day is better, as these fish graze all day long in the wild. A good choice for feeding tangs is “Nori,” which are sheets of dried seaweed that you can clip to your tank and the Tang will rip off pieces and eat them.